Abstract
Background: Lenalidomide, bortezomib and dexamethasone (RVD) was demonstrated to be an effective and well tolerated induction regimen in both transplant eligible and ineligible patients. Two large randomized phase III trials show an overall response rate (ORR) >95% (Durie et al, Attal et al) supporting the combination. We have evaluated our institutional data of patients treated with RVD induction therapy.
Methods: 751 newly diagnosed MM patients were treated with RVD induction therapy [R - 25 mg/day (days 1-14), V - 1.3 mg/m2 (days 1, 4 8, 11) and D - 40 mg once/twice weekly as tolerated every 21 days] from January 2008 until April 2015. Dose-adjustments were made based on the treating physicians discretion and patient tolerability. Demographic and outcomes data for the patients were obtained from our IRB approved myeloma database and responses were evaluated per IMWG Uniform Response Criteria.
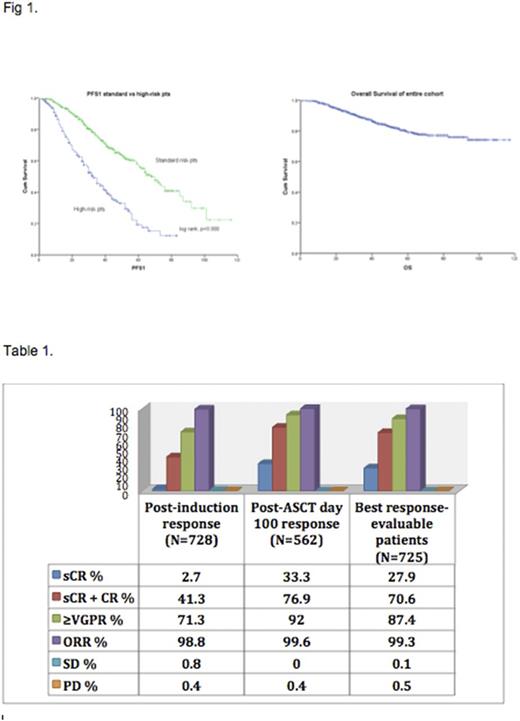
Results: The median age was 61 years (range 22-83). Other patient characteristics include: M/F 54%/46%; W/AA 58%/30%; ISS I/II/III 36%/24%/19%; Isotype IgG/IgA/FLC 56%/21%/17%; high risk/standard risk 28%/62%. Among the standard risk patients, t(11;14):13%, del (13): 30% comprised majority of the patients. Among high risk patients, the distribution of high risk cytogenetic abnormalities are t(4;14)/t(14;16)/del(17p): 5%/2.5%/10.5%. Complex karyoptye by conventional metaphase karyotyping was seen in 16% of the patients. A total of 562 patients (74.8%) underwent ASCT1 and 165 patients (22%) were offered deferred transplant. Among the patients that opted for deferred transplant, 56 patients (33.9%) received ASCT1 at first relapse. Median time to receive ASCT1 among these relapsed patients was 30 months (3-96 months). For others, median time for patients to receive ASCT1 was 5 months (range, 1-64 months). Post-ASCT, 70% patients were on maintenance therapy tailored to their risk (R-54%; RVD-8%; V-4%). This differed from the deferred ASCT patient population (mostly standard risk patients), where 66% of the patients received revlimid based maintenance. Median PFS1 among the deferred group was 73 months (range, 54.3-91.62). At a median follow up of 44 months for the entire cohort, the estimated median PFS was 56 months (49.85-62.15 months). Median PFS for standard risk patients was 68 months (60.62-75.37) versus 33 months for the high-risk patients (28-37.9 months), p<0.001 and median OS has not been reached, but encouraging results were seen for both high-risk and standard risk cohort with 64% and 77% of patients alive at the 84-month mark, respectively (Fig 1). Response rates are summarized in table 1.
Conclusions: This is the largest reported cohort of myeloma patients treated with RVD regimen and highlights the activity of this induction regimen with superior response rates of close to 90% ≥VGPR rates post-ASCT. This regimen should serve as an important bench mark for future studies designing optimal induction regimens.
Heffner: ADC Therapeutics: Research Funding. Boise: Abbvie: Consultancy; Eli Lilly and Company: Research Funding. Kaufman: Amgen, Novartis: Research Funding; Amgen, Roche, BMS, Seattle Genetics, Sutro Biopharma, Pharmacyclics: Consultancy. Nooka: Amgen, Novartis, Spectrum, Adaptive tecnologies: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal